The pRB/E2F pathway. The retinoblastoma gene, Rb, was the first tumor suppressor gene to be identified, and it has been a longstanding gene of interest for the Lees Lab. The protein product of this gene, pRB, is inactivated in the vast majority of human tumors through mutation of the Rb gene or its upstream regulators. pRB was originally identified as an inhibitor of cell cycle entry. It mediates this function, in part, by suppressing the activity of the E2F family of transcription factors, which drive expression of the core cell cycle regulators. Subsequent studies have linked pRB to a variety of other biological processes. It regulates terminal differentiation, by controlling cell proliferation and influencing fate determination via regulation of transcription factors that are master regulators of lineage specification. It also functions as both a repressor and activator of apoptosis, through transcriptionally dependent and independent mechanisms, depending on the cellular context. Additionally, pRB regulates cell metabolism and, in epithelial cells, controls cell polarity and migration. The Lees Lab has contributed to our understanding of pRB in each of these biological contexts. We continue to study the mechanisms of action and crosstalk of pRB, and the individual E2F family members and investigate how disruption of these events influences tumor development and metastasis.
Ongoing projects include:
- Understanding the consequences of Rb mutation on epithelial tumors, and
- Elucidating the non-canonical role of E2F4 in controlling multiciliogenesis.
CDK10/CycM and Primary Cilia. The CDK10/CycM complex was originally implicated in regulation of mitosis. However, subsequently studies revealed that CDK10/CycM regulates formation of primary cilia. Primary cilia are hair-like projections on the surface of a cell that receive signals from the surrounding environment and activate signaling pathways that influence cell states, proliferation and survival. The Lees Lab has shown that mutations in CDK10 or CycM are present in patients with ciliopathy disease syndromes. Moreover, we have linked primary cilia to stem cells in mammary tissues and tumors. Specifically, the lab has shown that the epithelial-mesenchymal transition (EMT) promotes formation of primary cilia on normal mammary stem cells and cancer stem cells in basal mammary carcinomas, and that primary cilia are essential for basal carcinoma formation.
Ongoing projects include:
- Elucidating the mechanisms that couple EMT to primary cilia formation, and
- Identifying the signaling pathways required for cilia-dependent tumor formation.
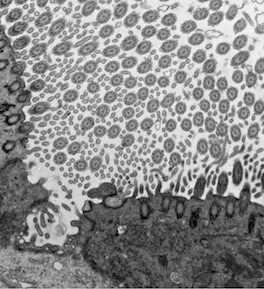
Ciliated cells in the nasal epithelium

